Chapter 1. Fundamentals
1.1 Moist thermodynamics 2/20
(Ref. 4, /chapter_4_Moist thermodynamic processes/4.4)
Moisture variables
Density (rv, rd)
Water vapor mixing (r): mass of water vapor per unit mass of dry air, r = rv/rd
Specific humidity (q): mass of water vapor per unit mass of air (including the vapor)
Vapor pressure (e or pv)
Partial pressure of dry air (pd)
Dalton’s law: p = e + pd
Rd : the weighted mean gas constant for all the constituents of air other than water vapor
Rv : the gas constant for water vapor
Relative humidity (RH): the ratio between the actual and saturation vapor pressure = e / e*
r = rv /rd = Rd/Rv e / (p-e), where e = Rd / Rv
q = rv / (rd + rv) = r / (1+r)
Saturation values, r*, q*, e*
Liquid water mixing ratio (rl)
Ice mixing ration (ri)
Thermodynamics of unsaturated moist air
The effective heat capacities of moist air are influence by the presence of water vapor.
Assuming the water vapor molecule is in the ground state
Cvv = 3 Rv = 1384.53 J kg-1 K-1
Cpv = 4 Rv = 1846.04 J kg-1 K-1
Over the range of tropospheric conditions
Cvv ~ 1410 J kg-1 K-1
Cpv ~ 1870 J kg-1 K-1
First thermodynamic law and second thermodynamic law: dQ = dU + pdV
Closed system and Open system
Cv¢ º (¶Q/¶T)v ~ Cvd (1+0.94r)
Cp¢ º (¶Q/¶T)p ~ Cpd (1+0.85r)
dQ = Cv¢ dT + pda
dQ = Cp¢ dT - a dp
specific volume: a º R¢ T / p
In adiabatic process,
d lnT = (R¢/Cp¢
) dlnp
Phase equilibrium of water substance
The discussion above focuses on a homogeneous system, where only a single phase is present. For such a system, thermodynamic equilibrium requires mechanical and thermal equilibrium: no pressure and temperature difference between the system and its environment. For a heterogeneous system, wherein multiple phases are present, thermodynamic equilibrium also requires chemical equilibrium: no diffusion of mass from one phase to another.
The phase equilibria of water substance are determined by requiring
Ti = Tii , pi = pii , gi = gii
where g is the Gibbs free energy: g º u + pa - Ts = k - Ts, s is the specific entropy,
k is the enthalpy: k º u + pa.
For a reversible, infinitesimal change while maintaining equilibrium,
dgi = dgii,
Since dg = du + p da + a dp - Tds- sdT, and Tds = du+ p da (first law of thermodynamics)
ai dp-si dT = aii dp-sii dT ® (dp/dT) i,ii = (sii- si)/(aii - ai)
The latent heat pertaining to the phase transition of a substance is defined as
L i,ii º (kii- ki) = T(sii- si)
(dp/dT) i,ii = L i,ii / T(aii - ai)
This is the Clausius-Clapeyron equation

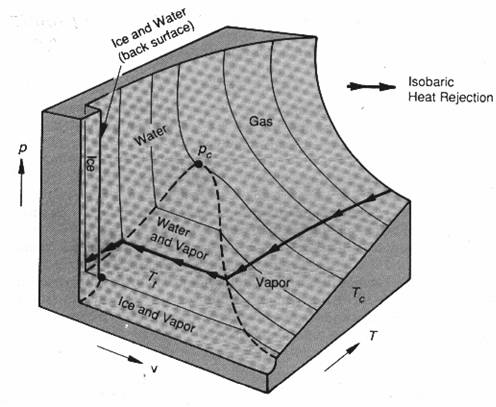
State surface for a (single-component) mixture of water involving multiple phases in equilibrium with one another. Thermodynamic process accompanying isobaric heat rejection also indicted.
Glaciers move (freezing decreases with increasing pressure)
rice
> rwater
Clausius-Clapeyron Equation 2/20
![]() ,
, ![]()
![]() ,
, ![]()
1) Water-vapor equilibrium
![]() ,
, ![]()
2) Ice-vapor equilibrium
![]() ,
, ![]()
3) Ice-water equilibrium
![]() ,
, ![]() but
but ![]()
Home work 1 (due 3/13):
From the
Clausius-Clapeyron Equation,
![]() ,
,
and the integrated
Kirchhoff equation,
![]() ,
,
derive the above
equations for
![]() ,
,
![]() ,
and
,
and
![]() -1.344x105
mb K-1.
-1.344x105
mb K-1.
Also plot e*(T).
|
|
|
Bergeron-Findeisen effect (Wegner- Bergeron-Findeisen)
Limitations of Clausius-Clapeyron Equation in cloud microphysics
Conserved moist thermodynamic variables
1.2 Clouds and microphysics
Characteristics and classification of clouds (student exercise)
Refer to Chapter 1: Identification of Clouds,
Houze, R. A. Jr., 1993: “Cloud dynamics”, Academic Press.
Homework 2 (due 3/20): translate description of clouds into English
Types of microphysical processes
Nucleation of particles
homogeneous and heterogeneous nucleation
Vapor diffusion
condensation, evaporation, deposition, and sublimation
Collection
continuous & stochastic coalescence, aggregation, riming, hail
Breakup of drops
Fallout
fall speeds of water drops and ice particles
Ice enhancement
several hypothesized processes
Melting
Refer to Chapter 3: Cloud microphysics,
Houze, R. A. Jr., 1993: “Cloud dynamics”, Academic Press.
1.3 Radiation 10/4
Refer to chapter_3: Atmospheric Radiative transfer and climate (3.1 to 3.8)
Dennis L. Hartmann, 1994: "Global Physical Climatology", Academic Press.
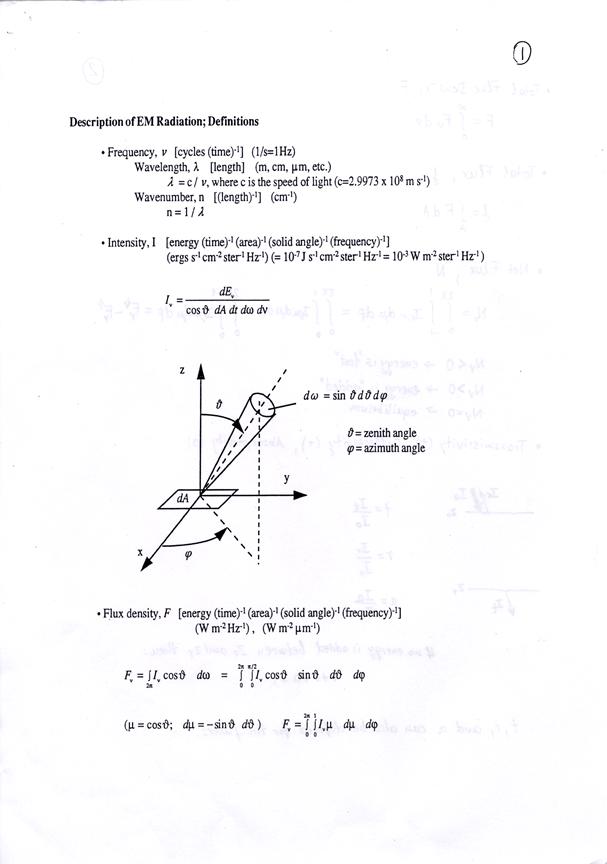
˙Total Flux Density, F
F
=
![]() F
F![]() d
d![]()
˙Total Flux,![]() f
f
f=![]() dA
dA
˙Net Flux, N
N![]() =
=![]() I
I![]()
![]() d
d![]() d
d![]() =
=![]() I
I![]()
![]() d
d![]() d
d![]() -
-![]() I
I![]()
![]() d
d![]() d
d![]() =F
=F![]() -F
-F![]()
![]()
N![]() < 0 → energy is “lost”
< 0 → energy is “lost”
N![]() > 0 → energy is “added”
> 0 → energy is “added”
N![]() = 0 → equilibrium
= 0 → equilibrium
˙Transmissivity (t), Reflectivity (r), Absorptivity (a)
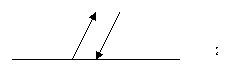 t =
t =
![]()
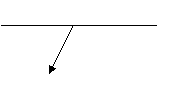 r =
r = ![]()
a =
![]()
If no energy is added between Z0 and Z1 then:
t + r + a = 1
t1r1 and a can also be defined for the fluxes.
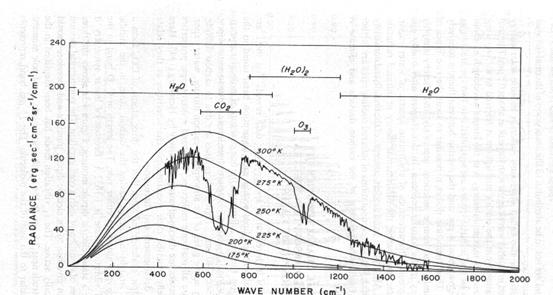
The terrestrial infrared spectra and various absorption bands. Also shown is an acture atmospheric emission spectrum taken by the Nimbus IRIS instrument near Guam at 15.1° N and 215.3°W on April 27, 1972.
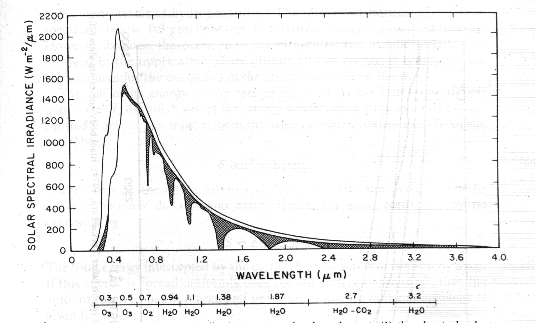
Spectral irradiance distribution curves related to the sun; (1) the observed solar irradiance at the top of the atmosphere, and (2) solar irradiance observed at sea level. The shaded area represent absorption due to various gases in a clear atmosphere.
3/6 Home work (due 3/20)
Planck function:
Bl(T) = 2 h n3 / [c2(exp(hn/KT)-1], h=6.6262x10-27 erg sec, K=1.3806x10-16 erg deg-1.
= 2 h c2 / l5 [c2(exp(hc/KlT)-1
Plot the plank function and derive the Stefan-Boltzmann Law (hint, x= hc/KlT
Interactions between em-radiation and matter
Matter in the atmosphere:
— gas molecules
N2 (78 %)
O2 (21 %)
CO2 (0.03 %)
H2O (0 ~ 0.04 %)
O3 (0 ~ 12![]() 10-4 %)
10-4 %)
others (10-4 ~ 10-9 %)
— aerosol
— cloud (water droplets, ice crystals)
Interactions:
— Scattering: only part of the e.m.-radiation travels in the original direction, some appear in directions other than the original one.
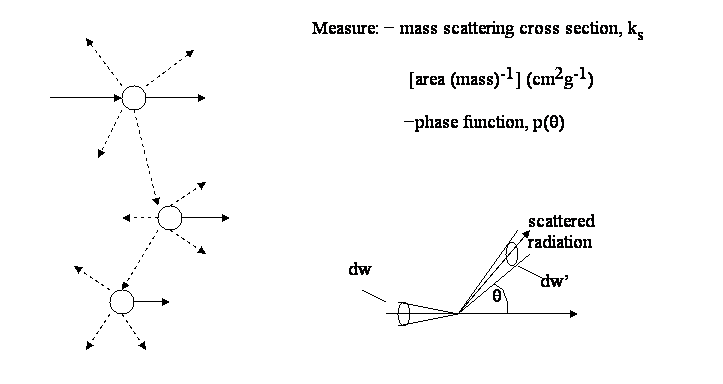
Is ~ ksI0dw
Is(q)
dw’ ~ ks![]() dw’ I0dw
dw’ I0dw
Is =
![]() Is(q)
dw’
Is(q)
dw’
![]()
![]() p(q)
dw’ = 1
p(q)
dw’ = 1
Types of scattering
Scattering depends on:
— chemical composition of the particle
— size of the particle, d
— wavelength of the incident radiation (λ)
1) d << λ → Rayleigh scattering (molecules)
ks ~ λ-4 (explains the blue color of the sky)

2) d
![]() λ → Mie scattering (aerosol, cloud)
λ → Mie scattering (aerosol, cloud)


— Absorption: some of the e.m. energy is converted into a different form of energy, e.g. kinetic energy, heat.
Measure: mass absorption cross section, ka
[area (mass)-1] (cm2g-1)
— Molecules
Energy forms of a molecule:

Etotal = Etranslational + Erotational + Evibrational + Eelectronic

Ev = nhv
h = 6.625![]() 10-34 Js
10-34 Js
(Planck constant)
Example: energy levels for electronic transitions:


Another way of describing e.m. radiation: consists of discrete amounts of energy called photons, Ev = hv
Absorption
occurs if the energy of incident photon has an energy equal to the difference
between two energy levels of the molecule. The molecule gets excited: Ei→Ej
, j > i. When Ej→Ei,
the molecule emits a photon with an energy of
![]() E=Ej-Ei.
Since
E=Ej-Ei.
Since
![]() E is
quantized, and equal to hv, the absorption and emission are selective.
E is
quantized, and equal to hv, the absorption and emission are selective.
Absorption and emission lines and line broadening

Broadening:
a) natural
b) pressure (collisions), Lorentz line shape
dominant in the troposphere
c) Doppler

Absorption/emission spectra:
combination of lines corresponding to different energy levels of different
transitions ![]() very complex structure →
bands
very complex structure →
bands
Main absorption features in the atmosphere:
H2O : triatomic molecule with permanent dipole moment →
— pure rotational band (l![]() 14 mm)
14 mm)
— strony vibration-rotation band at ~ 6.3mm
— several n/t bands between 0.8-4 mm
CO2: linear symmetric molecule → no permanent dipole moment →
no pure rotational band
— strong n/t band ~15mm (peak of the terrestrial radiation)
— several other bands at 2, 3 and 4 mm
O3: triatomic molecule
— strong n/t band at 9.6 mm
— weak band between 0.5-0.7 mm
N2 and O2: diatomic, symmetric molecules → no permanent dipole moment → no vibrational and simple rotational spectra.
Absorption and emission caused by electronic transitions. (high energy) → UV and visible spectra.
None or very weak absorption between 0.3-0.7 mm
Additional types of absorption
a) Photodissociation: breaking the bond between atoms.
Requires large energy → l < 0.2, 0.3 mm
b) Photoionization: removing electrons. Even larger energies are needed → l < 0.1 mm
Particles: energy levels are so complex, and so close to each other that absorption and emission is continuous.
Extinction) ke
ke= ka+ ks
The Equation of Transfer (E.T.)
The change of energy is due to extinction and emission:
dI = -kerdsI + jrds

![]() = -I + y, y =
= -I + y, y = ![]() : source function
: source function
Special case: assume y = 0
I(S1) = I(0) e-![]() (Beer-Bouguer-Lambert Law)
(Beer-Bouguer-Lambert Law)
Note: it is valid for fluxes too.
t =
![]() = e-
= e-![]()
E.T. for a plane-parallel atmosphere

Introducing the normal optical depth
as d![]() = -keρdz
= -keρdz
![]() =
=![]()
m![]() = I(
= I(![]() ) - y(
) - y(![]() )
)
Source function, y in Radiative Transfer Equation (RTE)
a) For multiple scattering:

jrds
= rds![]()
![]() (dw, dw’)I(dw’)dw’
(dw, dw’)I(dw’)dw’
y =
![]()
![]() (dw, dw’)I(dw’)dw’
(dw, dw’)I(dw’)dw’
![]() →
→![]() =
single scattering albedo
=
single scattering albedo
y depends on I (!!!)
![]() no “easy” solution for RTE.
no “easy” solution for RTE.
b) For thermal radiation:
jrds =
![]() rds
rds
![]() : intensity of radiation emitted at a wave length
l
by the atmosphere (or surface) at
temperature T.
: intensity of radiation emitted at a wave length
l
by the atmosphere (or surface) at
temperature T.
![]() =f(l, T)
in thermodynamic equilibrium f(l,
T) is the same for any substance (Kirchhoff’s Law)
=f(l, T)
in thermodynamic equilibrium f(l,
T) is the same for any substance (Kirchhoff’s Law)
for a “black body” al=1
![]() f(l,
T) is the emitted intensity of a black body, Bl(T)
f(l,
T) is the emitted intensity of a black body, Bl(T)
![]() = alBl(T)
→ y=
= alBl(T)
→ y=![]() =
=![]() = (1-
= (1-![]() ) Bl(T)
) Bl(T)
ke=1-![]() el=
emissivity
el=
emissivity
 =al
=al![]()
 = el
= el
el=
al
![]() emissivity = absorptivity
emissivity = absorptivity
Bl(T)=![]() Planck’s Law h=6.63
Planck’s Law h=6.63![]() 10-34Js
10-34Js
k=1.38![]() 10-23JK-1
10-23JK-1
Properties of B:
— Bl(T) does not depend on direction (isotropic radiation)
—
The wavelength of
maximum emission is inversely proportional to the absolute temperature:
lmax[mm]=![]()
(Wien’s law)
(Source function, y contiuned)
Black body curves for solar and terrestrial temperatures


Solar and terrestrial radiation can be treated separately
— total energy in a hemisphere (total flux density):
F= =sT4
(Stefan-Boltzmann law)
=sT4
(Stefan-Boltzmann law)
s=5.67![]() 10-8 Wm-2K-4
10-8 Wm-2K-4
Solution of the RTE in a plane-parallel atmosphere
RET:
m
![]() =I(τ;μ;φ)-y
(τ;μ;φ)
=I(τ;μ;φ)-y
(τ;μ;φ)

![]()
![]()

I(τ;μ;φ)=I(τ1;μ;φ)e-(τ1-τ)/μ
+  e-(τ'-τ)/ μ
e-(τ'-τ)/ μ
![]()
I(τ;-μ;φ)=I(0;-μ;φ)e-τ/μ
+  e-(τ-τ')/ μ
e-(τ-τ')/ μ
![]()
Total fluxes in the infrared (IR) spectrum (thermal flux)
1)
Assume there is no
scattering (![]() =0)
=0) ![]() y= Bl(T)
y= Bl(T)
2)
Use transmissivity, t
as a vertical coordinate in RTE instead of τ. t(z1,z2)=e
3)
Calculate F(![]() )=
)=![]()
Upward flux at the top of the
atmosphere (z=![]() ):
):
F↑(![]() )=
)=
Downward flux at the surface (z=zs):
F↓(zs)=

![]() =
=![]()
Plot of
![]() and T(z)
and T(z)

![]() − the transmissivity between surface and top of atmosphere −
is small.
− the transmissivity between surface and top of atmosphere −
is small.![]() Only small amount of energy from the surface reaches the top
Only small amount of energy from the surface reaches the top
![]() is large enough only for small values of z. Most of the flux
F↓ comes from the lower part of the atmosphere.
is large enough only for small values of z. Most of the flux
F↓ comes from the lower part of the atmosphere.
![]() In an absorbing atmosphere:
In an absorbing atmosphere:
1) energy loss by emission to space is much less
than the IR emission
![]() from the surface, “greenhouse”
effect
from the surface, “greenhouse”
effect
2) there is a supply of downward flux from the
warm lower atmosphere
Emission temperature, Te
F↑(![]() )=
)=![]() →Te=
→Te=![]() ≈ 255K or -18ºC
≈ 255K or -18ºC
Radiative equilibrium temperatures
Assume:
—Solar radiation is absorbed at the surface only,
—The atmosphere is composed of layers which emit like, black bodies, they are opaque for IR radiation
—There is a balance of incoming and outgoing fluxes for each layer.

Atop=0.3 (planetary albedo)
S=1360Wm-2 (solar constant)
Solution T1=255K (=Te)
T2=303K
Ts=335K (observed mean
Ts ≈ 288K (!)) ![]() there are physical processes other than radiation which
transport heat away from the surface (convection, evaporation)
there are physical processes other than radiation which
transport heat away from the surface (convection, evaporation)
Fact:
radiative equilibrium T(z)![]() observed T(z) → do convective adjustment:
observed T(z) → do convective adjustment:
![]() kntical value (6.5 K km-1). If
kntical value (6.5 K km-1). If
![]() >6.5K km-1 assume a nonradiative upward heat
transfer.
>6.5K km-1 assume a nonradiative upward heat
transfer. ![]() is lapse rate.
is lapse rate.
Radiative-Convective Equilibrium Temperature (RCET) profiles
— Obtained from a steady balance solution of complex models.
The models include the following variables.
• H2O
• CO2
• O3
• Aerosols
• Cloud
• Surface albedo
— RCET profiles approximate the global mean temperature profile of the Earth’s atmosphere.
Is the atmosphere-surface system in a radiative balance?

 Need
to calculate or observe the net flux at the top of the atmosphere:
Need
to calculate or observe the net flux at the top of the atmosphere:
![]()
![]() NTOA=NSW+NLW=F
NTOA=NSW+NLW=F![]() (1- ATOA)-σT
(1- ATOA)-σT![]() SW:
short wave
SW:
short wave
LW: long wave
NTOA averaged over
several years is zero![]() equilibrium.
equilibrium.
On a monthly time scale NTOA > 0 or NTOA< 0.
What about the radiative balance at the surface?
NSRF=NSW+NLW=F![]() (1-ASRF)-eσT
(1-ASRF)-eσT![]() + F
+ F![]()
Daytime: eσT![]() ≈ F
≈ F![]()
![]() the shortwave heating is dominant
the shortwave heating is dominant
At night: Nsw=0 and eσT![]() > F
> F![]()
![]() cooling
cooling
How does the temperature change in time?
Conservation of energy![]() absorbed radiation is converted into heat
absorbed radiation is converted into heat
Absorbed radiation is ∆N=N(z+dz)-N(z) ——— z+∆z
∆N=cpρ∆z![]() ——— z
——— z
t: time
cp: specific heat at constant pressure
![]() : radiative heating rate
: radiative heating rate
Radiative heating rate profile
![]() < 0 cooling (in the longwave spectrum)
< 0 cooling (in the longwave spectrum)
![]() > 0 warming (in the shortwave spectrum)
> 0 warming (in the shortwave spectrum)
In the stratosphere LW cooling by CO2 is compensated by SW warming by O3.
In the troposphere LW cooling by CO2 is balanced by SW warming by H2O. LW cooling by H2O is balanced by nonradiative processes (convective heat transfer from the surface). (H2O is the most important greenhouse gas.)
Effect of clouds on NTOA and NSRF
|
|
SW |
LW |
Net (SW+LW) |
|
TOA |
increase ATOA decrease NSW cooling |
decrease Te increase NLW warming |
cooling/warming |
|
Surface |
decrease F decrease NSW cooling |
increase F increase NLW warming |
cooling/warmin |
Cloud forcing, CF
CF = Nall-sky – Nclear-sky
In SW: Nall-sky < Nclear-sky
![]() CFSW < 0 → cooling
CFSW < 0 → cooling
In LW: Nall-sky > Nclear-sky
![]() CFLW > 0 → warming
CFLW > 0 → warming
In the tropics CF ≈ 0
At high latitudes CF < 0