
HLJ1 protein, which can be induced when macrophages are stimulated with lipopolysaccharide (LPS), helps the conversion of high-molecular-weight (HMW) misfolded IL-12p35 homodimers to IL-12p35 monomers. Bioactive IL-12p70 heterodimers, composed of IL-12p35 and IL-12p40 subunits, are released into the circulation by macrophages and thereby stimulate natural killer (NK) cells. Eventually, activated NK cells in the liver and spleen release IFN-γ in sufficient quantities to lead to organ damage and even death during sepsis.
Sepsis is the body’s life-threatening response to infection. It occurs when the body overreacts in a toxic manner, leading to internal tissue and organ damage and even death. One’s immune system usually serves to fight invaders, such as bacteria and viruses; sometimes, however, the immune system turns on itself instead of fighting the “invaders,” inducing a sepsis state. In intensive care patients, sepsis is the single most usual cause of death, despite timely delivery of hemodynamic, metabolic, ventilatory, and renal support.
Although new therapies have been developed and tested, such as direct anti-cytokines therapy, patients often still do not survive the ordeal. To lower the mortality rate of this disease, Dr. Kang-Yi Su and his team from NTU’s Department of Clinical Laboratory Sciences and Medical Biotechnology believe that they must first understand the reason for the immune regulation imbalance—the principal cause of organ failure and septic shock. Although the complexity of immune modulation posed great challenges to their research effort, Su and his team discovered an innovative method for tackling the disease, and their study and findings were published in eLife, a prestigious biomedical and life sciences journal.
In their research, Su and his team identified a heat shock protein 40, HLJ1, which emerged as a key factor in both innate and adaptive immunity. During lipopolysaccharide (LPS)-induced endotoxic shock, HLJ1 deficient mice showed reduced organ injury and IFN-γ (interferon-γ) dependent mortality. Leveraging single-cell RNA sequencing technology, Dr. Su characterized liver nonparenchymal cell populations under LPS stimulation and recorded that HLJ1 deficiency affected IFN-γ-related gene signatures in distinct immune cell clusters. HLJ1 deficiency also leads to reduced serum levels of IL-12 in LPS-treated mice, contributing to dampened production of IFN-γ in natural killer cells (NK) and not CD4+ or CD8+ T cells; as a result, improving the survival rate.
Adoptive transfer of HLJ1-deleted macrophages into LPS-treated mice resulted in reduced IL-12 and IFN-γ levels, which protects the mice from IFN-γ-dependent mortality. In the context of molecular mechanisms, HLJ1 is an LPS-inducible protein in macrophages and converts misfolded IL-12p35 homodimers to monomers, maintaining bioactive IL-12p70 heterodimerization and secretion, followed by massive IFN-γ production in NK cells.
Dr. Wei-Jia Luo, the first author of this study, remarked, “Our study showed that HLJ1 may serve as a molecular target for the development of novel antisepsis or immunomodulatory therapies.” Currently, there is no existing development of antibodies or small molecules that inhibit HLJ1 expression. To deliver more effective medications and treatment, Dr. Su and his team will continue to identify novel potential drugs for HLJ1 modulation.
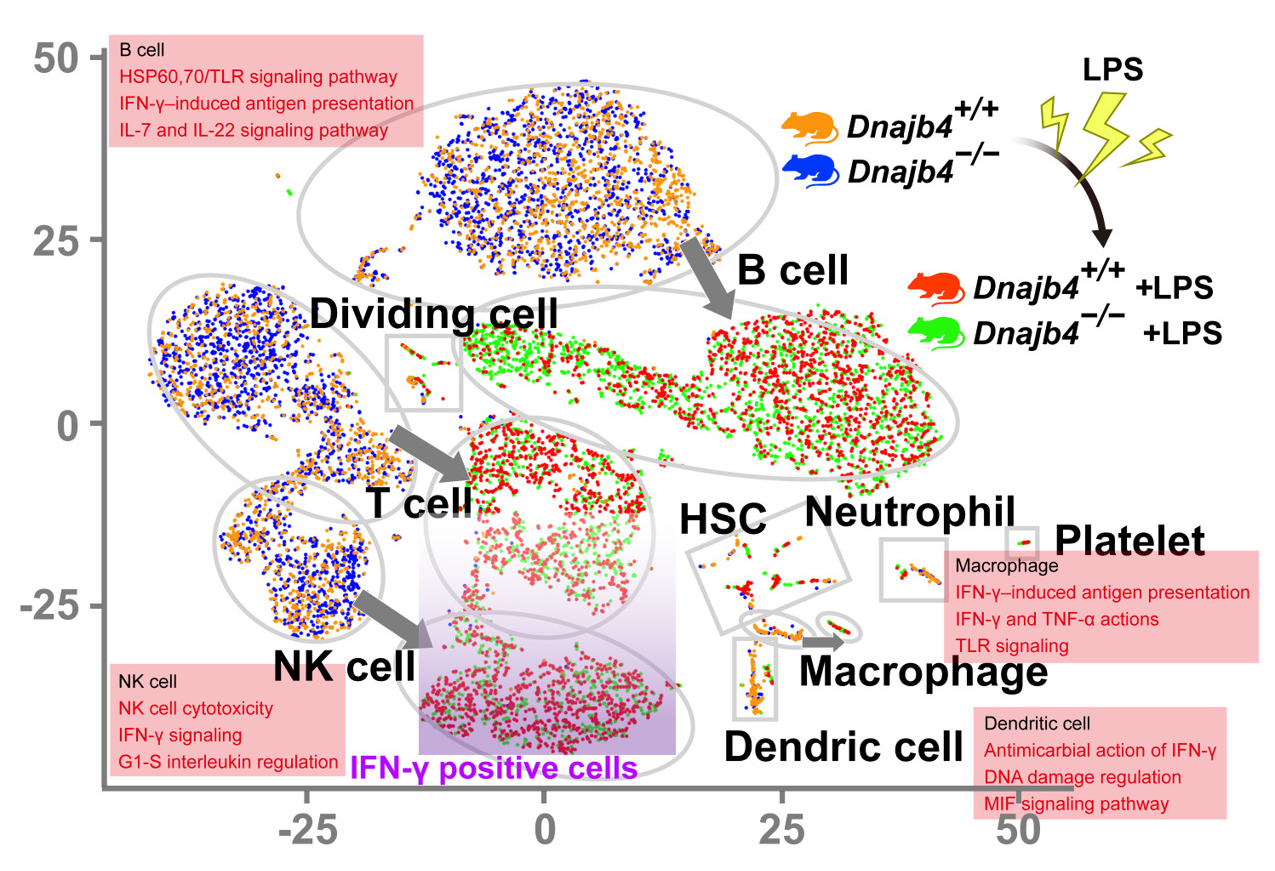
Single-cell RNA sequencing (scRNA-seq) reveals activated IFN-γ-mediated signaling pathways in macrophages and dendritic cells.

Dr. Kang-Yi Su and his research team from the Department of Clinical Laboratory Sciences and Medical Biotechnology.

The cover of eLife.

