|
Microflow Synthesis of Saccharide Nucleoside Diphosphate with Cross-coupling Reactions of Monophosphate Components
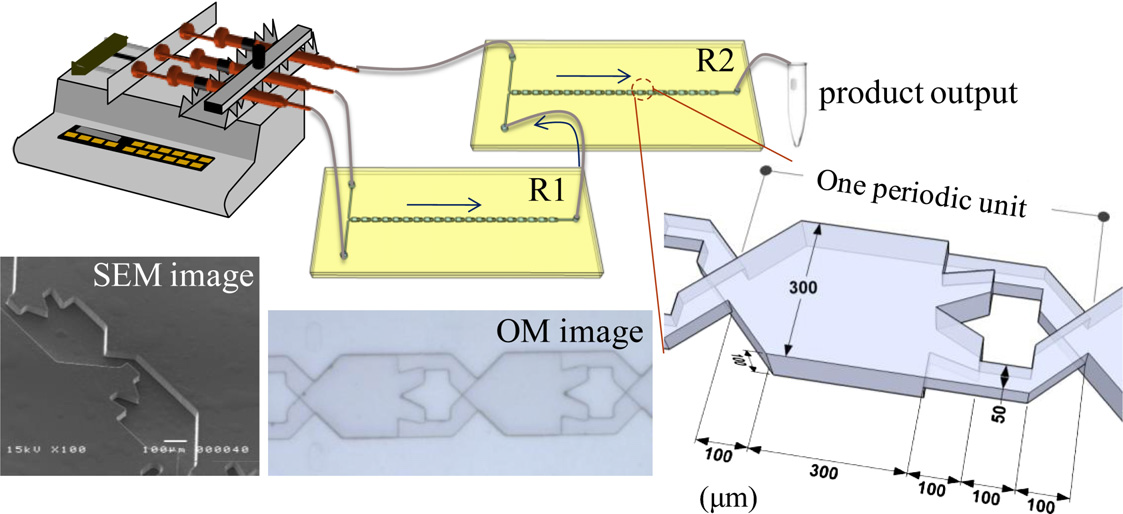
The advantageous features of a split-and-recombination (SAR) microreactor for organic synthesis of labile bioactive compounds are demonstrated with rapid and efficient cross-coupling reactions of two monophosphate components to form a saccharide–nucleoside diphosphate. With this SAR microreactor, 85 % conversion of a cross-coupling reaction (GlcNAc monophosphate reacting with UMP-morpholidate) to the diphosphate (acetylated UDP-GlcNAc) was achieved in 10 s, which is much less than a reaction period two days for 80 % conversion with a conventional batch reactor; the duration of reaction is decreased >105 fold. An abbreviated period and satisfactory output is expected to be realized when several SAR microreactors are applied in parallel.Chem. Eng. J. 2012, 198–199, 33–37. Flash Synthesis of Carbohydrate Derivatives in Chaotic Microreactors An efficient and rapid synthesis of carbohydrate derivatives was accomplished using a chaotic microreactor. The reactor shaped as split-and-recombine (SAR) structure which combines mixing mechanisms of both multi-lamination (diffusion) and chaotic advection to enhance mixing and reaction. Using two steps reaction process in SAR-microreactors, the carbohydrate derivatives, aldo-naphthimidazoles were generated by linkage of naphthalenediamine with mono-, di- or trialdoses in less than 10 s with satisfactory yield. Chem. Eng. J. 2011, 174, 421–424. Diphosphate Formation
Using Cyanuric Chloride or Triisopropylbenzenesulfonyl Chloride as
the Activating Agents
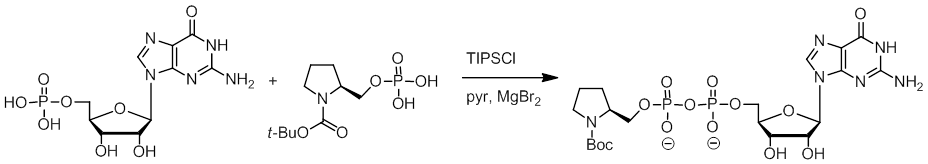
By using cyanuric chloride or 2,4,6-triisopropylbenzenesulfonyl chloride (TIPSCl) as the condensing agents and magnesium bromide as the promoter, the phosphate cross-coupling reactions are effectively carried out to give lipid–saccharide and pyrrolidine–guanosine diphosphates. Under optimized conditions, the TIPSCl-facilitated cross-coupling reactions complete in a short reaction time (~ 5 h) without complication of possible self-coupling reactions. Tetrahedron Lett. 2011, 52, 2232–2234. Tagging Saccharides
for Signal Enhancement in Mass Spectrometric Analysis
Oligo- and
polysaccharides are less sensitive in MS analysis partly due to
their neutral and hydrophilic nature to cause low ionization
efficiency. In this study, four types of
oligosaccharides including aldoses, aminoaldoses, alduronic acids
and α-keto acids were modified by appropriate tags at the reducing
termini to improve their ionization efficiency. Bradykinin (BK), a
vasoactive nonapeptide (RPPGFSPFR) containing two arginine and two
phenylalanine residues turned out to be an excellent MS signal
enhancer for maltoheptaose, GlcNAc oligomers and oligogalacturonic
acids.
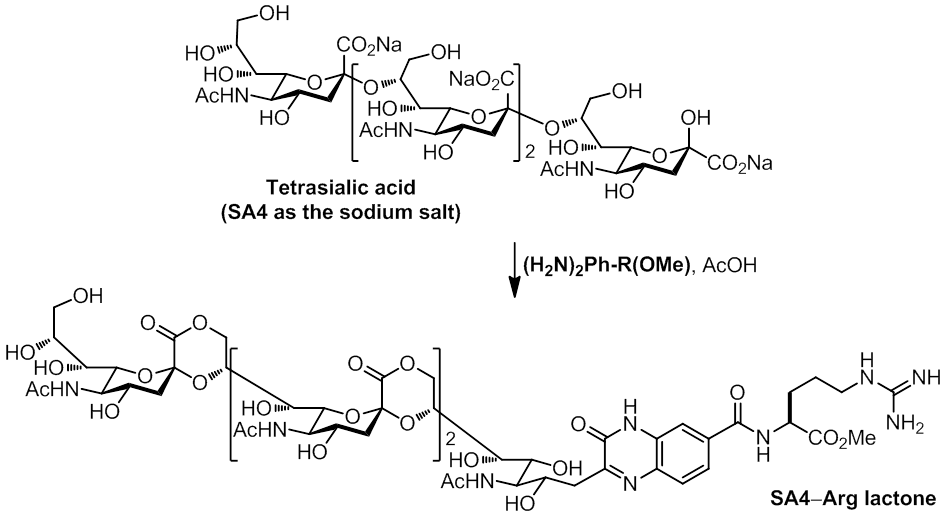
Interestingly, concurrent condensation and
lactonization
of
a2,8-linked
tetrasialic acid (SA4) was carried out
with
an arginine linked phenylenediamine (H2N)2Ph-R(OMe)
to obtain a quinoxalinone derivative, which showed >200-fold enhancement
over unmodified SA
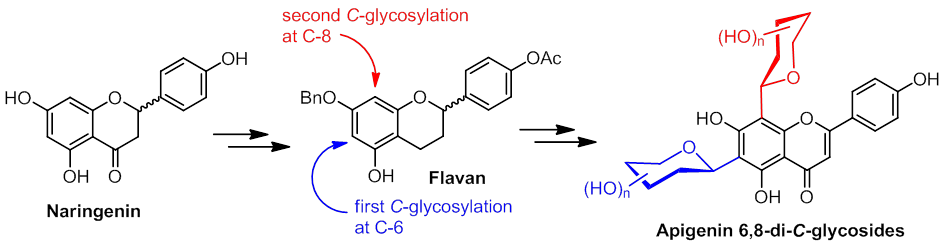
Regioselective
Synthesis of Di-C-glycosylflavones
Possessing Anti-inflammation Activities.
A series of 6,8-di-C-glycosylflavones
bearing identical or distinct glycosyl moieties are synthesized and
shown to exhibit anti-inflammation activity. Three methods are
utilized to synthesize a variety of 6,8-di-C-glycosylflavones
bearing identical or distinct glycosyl moieties. Some C-glycosylation
compounds are found to have better anti-inflammation activities than
the parent flavones. Among them, 6,8-di-C-glucosylapigenin
(known as vicenin-2) shows inhibition of TNF-α expression and NO
production with IC50 values of 6.8 and
Direct Amidation of Aldoses and Decarboxylative Amidation of α-Keto Acids: An Efficient Conjugation Method for Unprotected Carbohydrate Molecules
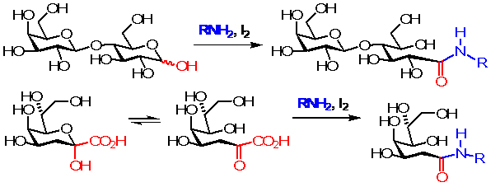
Using iodine as an appropriate oxidant, unprotected and unmodified aldoses undergo oxidative amidation with a variety of functionalized amines, α-amino esters, and peptides, whereas KDO, sialic acid and other α-keto acids proceed with oxidative decarboxylation followed by in situ amidation. Glycoside bond and many other functional groups are inert under such mild reaction conditions. This reaction protocol for direct ligation of carbohydrate molecules looks promising in development of a general and efficient synthesis of glycoconjugates. J. Org. Chem.2009, 74, 1549-1556.
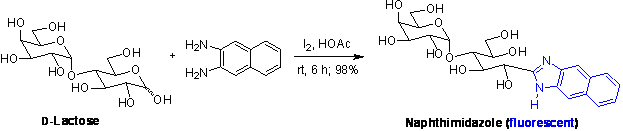
Using Molecular Iodine in Direct Oxidative Condensation of Aldoses with Diamines: An Improved Synthesis of Aldo-benzimidazoles and Aldo-naphthimidazoles for Carbohydrate Analysis
A practical method is developed for conversion of unprotected and unmodified aldoses to aldo-benzimidazoles and aldo-naphthimidazoles. Using iodine as an oxidant or promoter in acetic acid solution, a series of mono-, di- and trialdoses, including those containing carboxyl and acetamido groups, undergo an oxidative condensation reaction with o -phenylenediamine or 2,3-naphthalenediamine at room temperature to give the aldo-benzimidazole and aldo-naphthimidazole products in high yields. No cleavage of the glycosidic bond occurs under such mild reaction conditions. The composition analysis of saccharides is realized by the HPLC analysis of the fluorescent naphthimidazole derivatives. J. Org. Chem.2008, 73, 3848-3853..
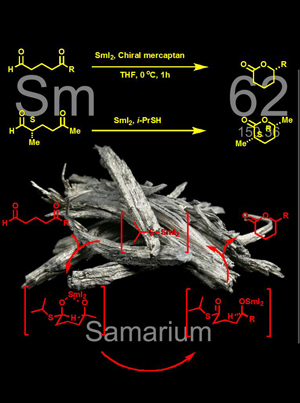
Stereoselective Synthesis of δ -Lactones from 5-Oxoalkanals via One-Pot Sequential Acetalization, Tishchenko Reaction, and Lactonization by Cooperative Catalysis of Samarium Ion and Mercaptan
By the synergistic catalysis of samarium ion and mercaptan, a series of 5-oxoalkanals was converted to (substituted) δ -lactones in efficient and stereoselective manners. This one-pot procedure comprises a sequence of acetalization, Tishchenko reaction and lactonization. The deliberative use of mercaptan, by comparison with alcohol, is advantageous to facilitate the catalytic cycle. Such samarium ion/mercaptan cocatalyzed reactions show the feature of remote control, which is applicable to the asymmetric synthesis of optically active δ -lactones. J. Org. Chem. 2001, 66, 8573–8584. |
|||